Next-Gen Radiology
AI algorithms and guided workflows deliver faster, more accurate cancer reads.
For radiologists, cancer analysis and reporting represents one of the most complex, time-intensive tasks in their day. AI Metrics revolutionizes cancer patient evaluation – by using artificial intelligence to improve the efficiency and accuracy of cancer reads.
With AI Metrics, you can:

Save Time
Reading and diagnostic image interpretation for a single cancer patient can take a radiologist 20 minutes or more. In a recent multi-institutional comparative effectiveness study, AI Metrics was found to reduce the average read time from 18.7 minutes to just 9.8 minutes compared to manual methods.
Prevent Burnout
By reducing the time and effort it takes to perform cancer analysis and reporting, AI Metrics can improve load balancing – helping radiologists get more done in less time. This helps alleviate burdens associated with radiologist burnout and staffing shortages.

Improve Accuracy
With guided workflows, rapid review of prior and preliminary reads, and automatic tracking of incidental findings, AI Metrics helps eliminate major errors.
Enhance Reporting
Each AI Metrics report is generated in the background, virtually worry- and error-free, allowing radiologists to keep their eyes on the images. Our clear, visualized reports are preferred by 100% of oncologists over standard text-based reports.

A better radiology workflow
A multi-institutional comparative effectiveness study compared AI Metrics to current manual methods. The study included 24 independent radiologists and 20 independent oncologic providers. See the highlighted results below.
2x faster reads
Reduced read time average from 18.7 minutes to just 9.8 minutes.
25% more accuracy
Improved reporting accuracy from 73% to 91%.
58% more consistency
Increased inter-observer agreement from 46% to 73%.
How It Works
AI Metrics helps radiologists cut read times in half, following this simple process:
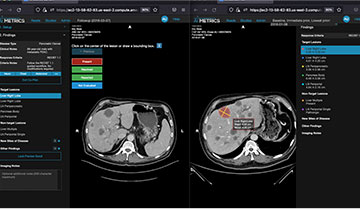
1. Activate Co-Pilot
To start a follow-up cancer patient evaluation, the radiologist activates AI Metrics Co-Pilot. With a single click, this AI-assisted system provides a rapid review of the patient’s prior and preliminary CT or MRI reads – automatically taking the radiologist to the image of each tumor being tracked in succession.
2. Measure Tumors
As each successive tumor target is displayed, the radiologist confirms that the right image has been selected. With a single click, the system automatically measures the target, applies all the prior labeling information, and calculates the percentage changes. Clicking the “next” button repeats this process for each target in the image exam, so the radiologist never has to take his or her eyes off the patient.
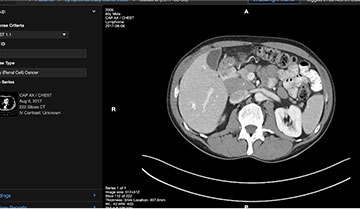
3. Track Incidental Findings
For lesions that are being tracked but not measured, the radiologist can quickly make an assessment by comparing the image to prior exams. Where necessary, areas of potential concern can be identified for follow-up, ensuring the finding is evaluated.
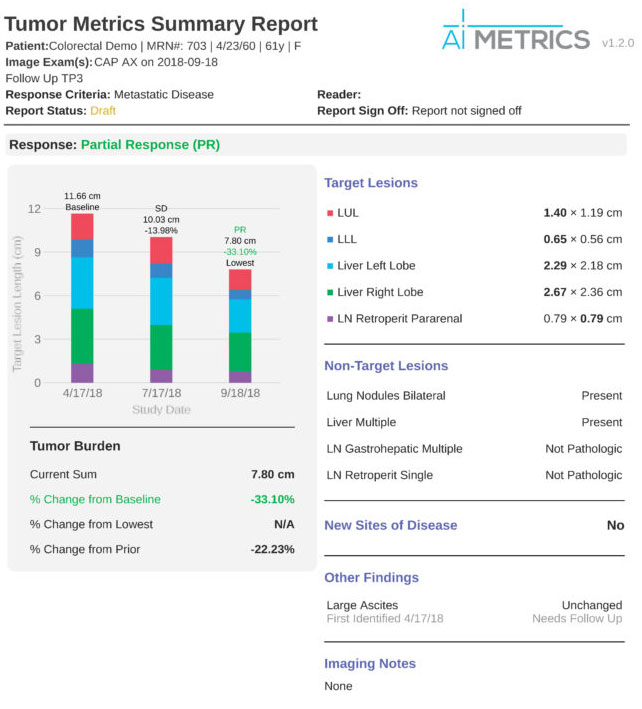
4. Generate Patient Report
As the radiologist evaluates patient images, AI Metrics works in the background to simultaneously create a clear, accurate, visualized report. This report automatically generates graphs, tables, images, and text – providing oncologists and patients with a concise overview of the patient’s current condition.
Watch AI Metrics In Action
Implementation is Easy
Software implementation is easy with AI Metrics. Our team will work alongside your IT staff to ensure fast, simple integration with your existing systems. With AI Metrics, you’ll benefit from:
Simplify Cancer Reads with AI Metrics
Ready to reduce radiologist burnout while delivering better, faster reads? Schedule a software demo today.
